

When periodic table elements or their compounds are heated on a flame or an electric arc or spark they emit energy in the form of light. Emission SpectroscopyĮmission spectroscopy is a spectroscopic method that examines the wavelengths of photons emitted by atoms or molecules during their transition from an excited state to a ground state or lower energy state. Therefore, absorption involved a transition from the ground state to a higher energy state.
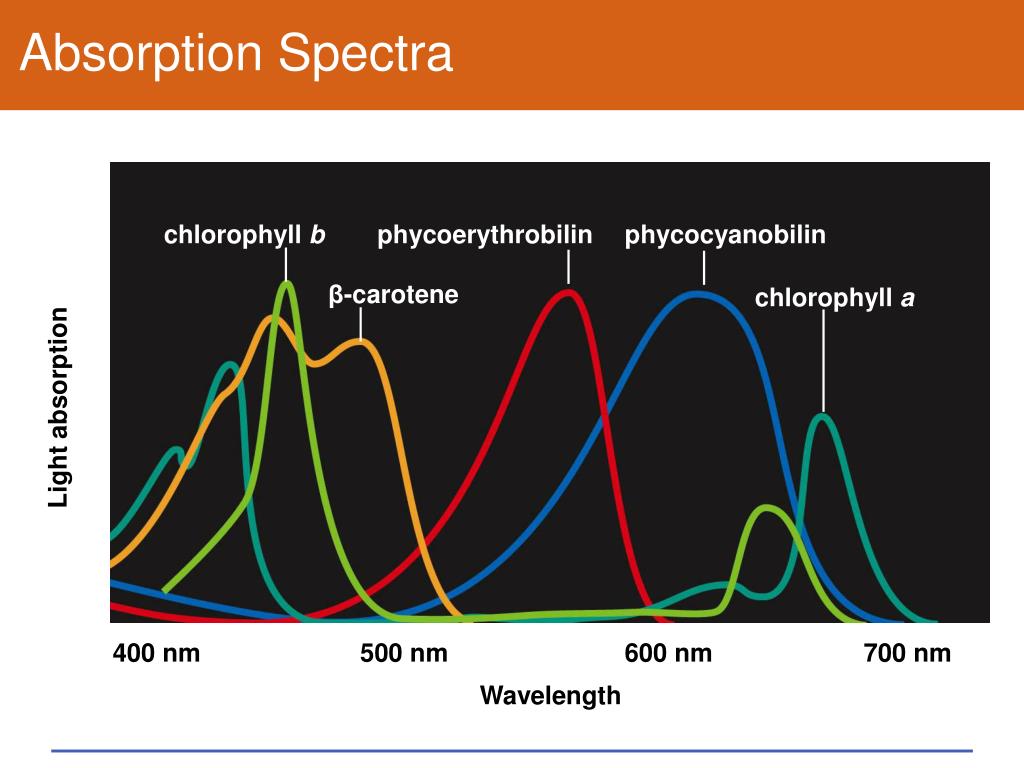
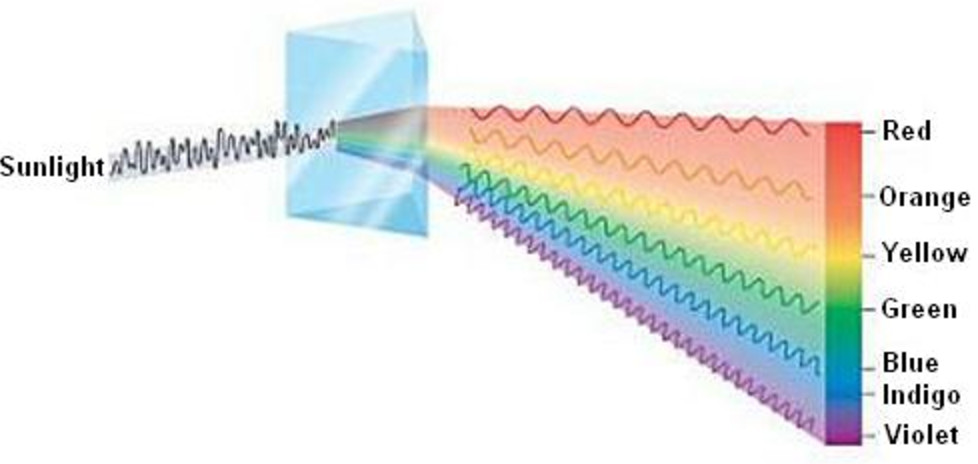
Electromagnetic energy is transferred to the atoms or molecules present in the analyzing sample and particles are promoted from lower energy states to higher energy states or exited states. When a beam of electromagnetic radiation passes through a chemical sample, a part of the radiation is absorbed by the sample. It is dependent upon the physical states of matter, the environment of absorbing species, and other related factors. Such spectra may be due to atomic or molecular absorption. The study of the frequency or wavelength of absorbed radiation provides absorption spectra of the analyzing sample. The sample absorbed radiation from continuous sources.Ībsorption spectroscopy is a spectroscopic technique that measures the absorption of radiation due to its interaction with a sample.There are two common ways in which the interaction is observed, Therefore, the particles are promoted from lower to higher energy states or exited states. Electromagnetic energy is transferred to the atoms or molecules present in the samples. When a beam of electromagnetic radiation is passed through a chemical sample, a part of the radiation may absorb by the sample. The instrument which records this variation in intensity is known as a spectrometer. After the interaction, there occur variations in the intensity of electromagnetic radiation over a certain range of frequencies or wavelengths. Spectroscopy is mainly concerned with the interaction of electromagnetic radiation with matter.


 0 kommentar(er)
0 kommentar(er)
